
On which block Hydrogen element is present? ġ) Hydrogen combine react with chlorine gas at room temperature form hydrochloric acidĢ)Hydrogen combine with oxygen molecule in air form water moleculeģ) It is mainly use to formation of ammoniaĤ) It is also use in agriculture Hydrogen is use to produce fertilizer productsĥ) It is use in purification of petroleum from ores Due to this nature hydrogen atom show valency 1. It is unpaired electron and easily lose or gain 1 electron to form stable compound. The electronic configuration in concert of shell is 1 The electronic configuration of hydrogen is 1S 1 ġ) H 1 is known as protium (0 neutron is present only 1 proton is present)Ģ) H 2 Is called as deuterium (1 neutron and 1 proton is present in nucleus)ģ) H 3 is called as tritium ( 2 neutron and 1 proton is present in nucleus ) Isotopes : hydrogen atom show 3 isotopes H 1, H 2, H 3. The properties of hydrogen atom is different from the all the elements of periodic table so it placed at a first position of periodic table. Melting point : hydrogen melt at the point of -259.20 c (The temperature at which solid converted to liquid ) Position : it is placed in periodic table at 1 st column and 1 st row. īoiling point : hydrogen boil at the temperature of -252.8 0 c (the temperature at which liquid covered gaseous form is called boiling point) Answer (1 of 3): The atomic number of an element is determined by the number of protons in the nucleus of its atom. Taste : it is tasteless and odourless gas. due to easily formation of covalent bonding with other H atom. Nature : it is found in gaseous form In nature hydrogen present in in H 2 molecule. Do not present any neutron Hence it show atomic weight is 1 (Present 1 proton and 1 neutron)Ītomic weight : hydrogen atom show atomic weight is 1.0079 In hydrogen only 1 proton is present. The meaning of this word is water making agentĪtomic symbol : it is represented by the symbol HĪtomic number : the atomic number of hydrogen is 1. Origin of name : the name hydrogen is created from Greek word hydro and genes. ĭiscovery : It is discover in 1766 by scientists Henry canvendish. Does not present in monoatomic from because hydrogen atom form easily bond with other molecule. In nature hydrogen only present in the form of H 2 molecule.
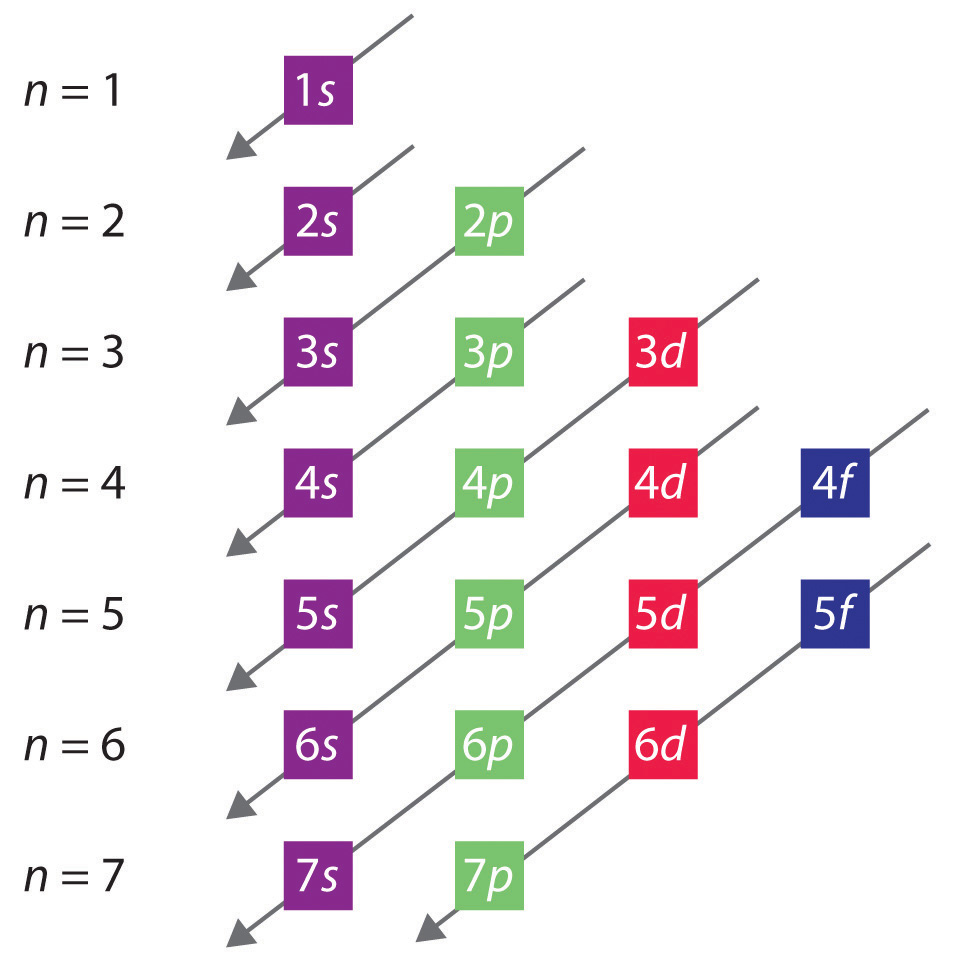
Electron configuration through orbit (Bohr principle) Electron configuration through orbital (Aufbau principle) Electron configuration through orbitals follows different principles. Electron configuration can be done in two ways. Which smallest element having only 1 electron. The electron configuration of hydrogen is 1s 1, if the electron arrangement is through orbitals. Hydrogen is the first element of periodic table. Atomic Mass, Number, Physical, Chemical properties, Electronic configuration, Valency, Chemical reaction, Uses Hydrogen – Learn all details regarding Hydrogen in Periodic Table i.e.


 0 kommentar(er)
0 kommentar(er)
